Introduction
Anti-BCMA(B-cell maturation antigen) CAR-T therapy, a novel and efficacious method for treating relapsed/refractory multiple myeloma (RRMM), has been approved and applied in clinics. Here, we report the survival analysis and clinical characteristics associated with long-term survivors (complete response at 18 months) after CAR-T cell therapy.
Methods
Between 1 January 2018 and 1 February 2023, 22 consecutive patients with relapsed/refractory MM were enrolled in a clinical trial of BCMA CAR T cells (registered at www.clinicaltrials.gov as #NCT04500431). This study was approved by the Tongji Hospital of Tongji University Ethics Committee, and written informed consent was provided by each patient. They all were treated with a single infusion of CAR-T cells. FC chemotherapy regimen was administered before transfusion. The median number of CAR-T cells transfused was 5.0 (range 4.0-6.6) ×10 6/kg. Long-term efficacy and related clinical characteristics were evaluated.
Results
In the 22 patients treated, the overall response rate was 77% (17 of 22). With a median follow-up of 24 months (range, 4-52), the median PFS were 17 months. The adverse events within 30 days after infusion were well tolerated. Toxic hematologic effects were the most common grade ≥3 events, including neutropenia (68%), anemia (54%), and thrombocytopenia (36%).Only 1 patient experienced grade 1 neurologic toxicity.No patients experienced severe infection within 30 days after infusion. Detailed patient baseline characteristics are described in Tables 1.
During follow-up after CAR T-cell therapy, the complete response rate was 36.3%(8/22), 40.9(9/22) and 27.2(6/22) at 12 months, 18 months and 24 months, respectively. At 18 months, the patients achieved the highest complete response rate. Baseline characteristics, such as age, sex, isotype, ECOG, high-risk cytogenetics, prior lines of therapy, percentage of plasma blasts in bone marrow, serum monoclonal protein and extramedullary disease in the 9 complete response patients at 18 months were similar to other progressive disease 13 patients. However, the significant differences of International Staging system stage(p=0.034) and Durie-Salm staging system(p=0.002) at baseline were observed between the 9 complete response patients and 13 progressive disease patients at 18 months(Table 1). The International Staging system stage(area under curve 0.80) and Durie-Salm staging system(area under curve 0.86) predicted the outcome of patients at 18 months well.
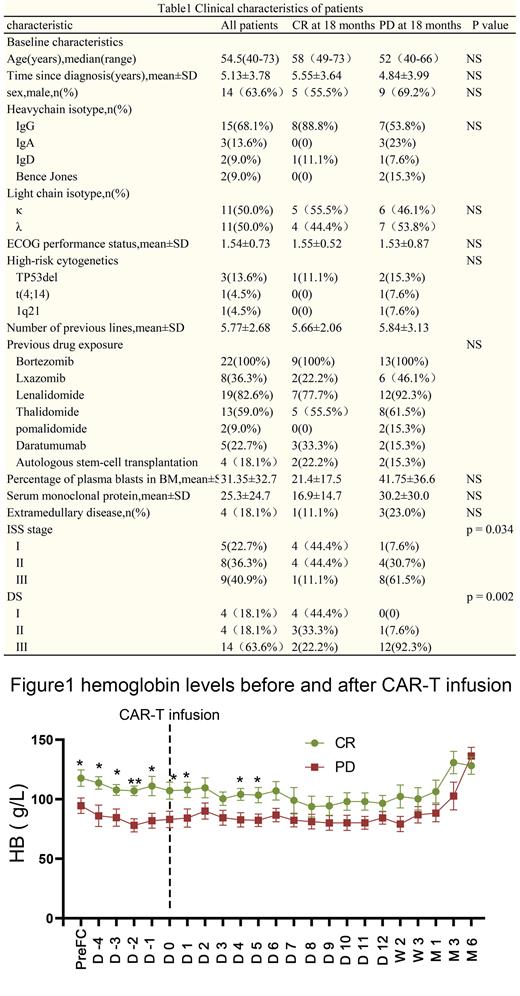
Durie-Salmon Staging system was published in 1975, which used commonly available clinical parameters including calcium, hemoglobin, bone lesions, creatinine level and serum or urine monoclonal protein. The ISS staging system was proposed in 2005 based on baseline serum levels of beta2-microglobulin (β2M) and albumin. And there was no statistically significant difference in the baseline serum levels of beta2-microglobulin, albumin, calcium, bone lesions and creatinine level between the 9 complete response patients and 13 progressive disease patients at 18 months. As figure 1 illustrates, the hemoglobin of 9 complete response patients was higher than that of 13 progressive disease patients with statistical significance at 9 time-points: PreFC(117.6±20.7 vs 94.6±23.6 , p = 0.028), D -4(113.6±15.9 vs 86.0±25.7 , p = 0.016), D -3(107.8±11.8 vs 84.6±23.0 , p = 0.027), D -2(117.6±20.7 vs 94.6±23.6 , p = 0.0009), D -1(111.0±19.9 vs 81.8±19.0 , p = 0.013), D0(107.1±20.4 vs 83.0±22.8 , p = 0.029), D1(107.7±19.1 vs 84.2±24.3 , p = 0.032), D4(104.1±14.9 vs 82.7±19.9 , p = 0.015), D5(103.4±19.1 vs 82.2±18.3 , p = 0.018). In the univariate Cox regression analysis, the hemoglobin at PreFC(hazard ration (HR) = 0.97; 95% confidence interval (CI): 0.95-1; p = 0.029), D -4(HR=0.96 ;95% CI:0.92-1, p = 0.012), D -3 (HR=0.97 ;95% CI:0.94-1, p = 0.029), D -2(HR=0.93 ;95% CI:0.89-1, p = 0.003), D -1(HR=0.97 ;95% CI:0.95-1, p = 0.047), D0(HR=0.97 ;95% CI:0.94-1, p = 0.038), D1(HR=0.97 ;95% CI:0.94-1, p = 0.044), D4(HR=0.95 ;95% CI:0.92-1, p = 0.01) and D5(HR=0.97 ;95% CI:0.94-1, p = 0.027) were relative factors of prognosis.
Conclusions
These long-term follow-up data in patients after BCMA CAR -T therapy suggest that baseline ISS ,DS staging and hemoglobin at 9 time-points display opposing association with long-term progression-free survival.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal